Thinking about Q(heat), W(work) and ΔE for Ideal Gas
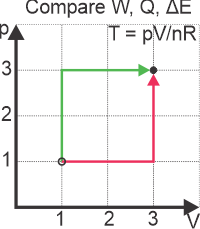

It is very important to think about the process and the signs involved or you will end up adding where you should be subtracting and subtracting where you should be adding. This is especially the case when you are moving around the pV diagram for ideal gases. Lets compare the work, heat and changes in thermal energy for an isochoric expansion followed by an isobaric process with one in which the isochoric follows the isobaric process.
PowerPoint Presentations Ref: Knight vs 4 Ch 19
- 3. Specific Heat and Calorimetry
- 4. Heat Transfer Mechanisms
Must See (Thermodynamics) Example Question
SolveIt! Nitrogen is stored in a high pressure gas cylinder (a standard cylinder holds 50 litres of compressed gas) at 1000 psi (70 atm) at room temperature (30 C) in my lab. A student (who shall remain nameless) opened the main valve allowing the gas to expand rapidly to room pressure (1 atm). (a) What is the resulting volume and temperature of the gas (and room)? (b) At constant pressure, the temperature of the gas slowly rises to room temperature (30 C). What is the final volume of the nitrogen gas? (有一瓶高壓的氮氣(V=50公升,P=70atm/1000psi)放在30度C的實驗室中,有一個學生把氮氣都放出來了,使所有空氣與實驗室的空氣(1atm)混和在一起
(a)請問最後實驗室與實驗室的空氣的溫度與容量會變成怎樣?
(b)請問"氮氣"的容量最後會變成多少 )
Question Paper (pdf)
FAQ

- Q: What is the difference between internal heat of a system and internal energy?
ANS: There is no such thing as internal heat for a system. Heat and Work are the means in which energy
is transferred into or out of a system. Just as you cannot say a system has 100 J of work, you cannot say it has
100 J of Heat. The internal energy of a gas can be thought as follows.
Each molecule has its own translational, rotational, vibrational, electronic and potential
energy. Each of these change with time. For simplicity we add all these energies together and then sum over all the
molecules of the gas call it the internal energy of the system. We do this because we are, in general not concerned
about changes in the energy of individual molecules but rather changes in the energy of the whole system.

 It is very important to think about the process and the signs involved or you will end up adding where you should be subtracting and subtracting where you should be adding. This is especially the case when you are moving around the pV diagram for ideal gases. Lets compare the work, heat and changes in thermal energy for an isochoric expansion followed by an isobaric process with one in which the isochoric follows the isobaric process.
It is very important to think about the process and the signs involved or you will end up adding where you should be subtracting and subtracting where you should be adding. This is especially the case when you are moving around the pV diagram for ideal gases. Lets compare the work, heat and changes in thermal energy for an isochoric expansion followed by an isobaric process with one in which the isochoric follows the isobaric process.
